Improving Access to Essential Asthma Medicines
One-third of the world’s population does not have access to essential medicines and every year out-of-pocket expenditures for medicines force approximately 100 million people into poverty. The causes for poor access to medicines are known and can be addressed.
A health systems perspective on essential asthma medicines
To fully understand access to essential asthma medicines we must take a health systems perspective. Poor access can be caused by many health systems issues, such as: high prices; resistance to supplying generic medicines; poor procurement procedures; health and pharmacy system problems; interrupted medicine supplies; slow registration of new medicines; outdated regulations and policies; and undue influence by pharmaceutical companies.
Robust medicine policies are key
Pharmaceutical pricing and reimbursement policies are key to achieving equitable and affordable access to medicines. Affordability and access are better in countries where prices are controlled. To improve access to essential asthma medicines, countries should seriously consider the following policy interventions recommended by the World Health Organization’s (WHO’s) ‘Guideline on country pharmaceutical pricing policies’: regulating the application of mark-ups across the pharmaceutical supply chain; exempting essential medicines and active pharmaceutical ingredients from taxes; using reference pricing to reduce price variability among comparable products; tendering; pooled procurement; and the use of quality-assured generic medicines. WHO’s Global Action Plan for non-communicable diseases (NCDs), extended from 2020 to 2030, has an 80% availability target for NCD essential medicines.
How easy is it to access essential asthma medicines in Africa?
Recent surveys in selected African countries reflect ongoing problems of access to essential asthma medicines.
The Gambia
A questionnaire in the Gambia gathered responses from the central medical stores (CMSs) that supply public health facilities and 8 registered private pharmacists who supply 19 of the 26 registered private pharmacies in the country. Most reported little availability of essential asthma medicines, and if one was available it was unaffordable. The only inhaled corticosteroid (ICS) available at the CMSs was beclometasone 50 mcg. Only 1 of 8 private pharmacists had a WHO-recommended ICS. Although inhaled salbutamol was available in the CMSs and through 6 private pharmacists, 7 of the 8 private pharmacists did not have high dose ICS inhalers or the WHO-recommended ICS-long-acting β2-agonist (ICS-LABA) combination inhaler of budesonide and formoterol (Chapter 13). The 8th pharmacist had beclometasone 50 mcg at a cost equivalent to 15 days’ wages and budesonide 100 mcg at a cost equivalent to 28 days’ wages.
Nigeria
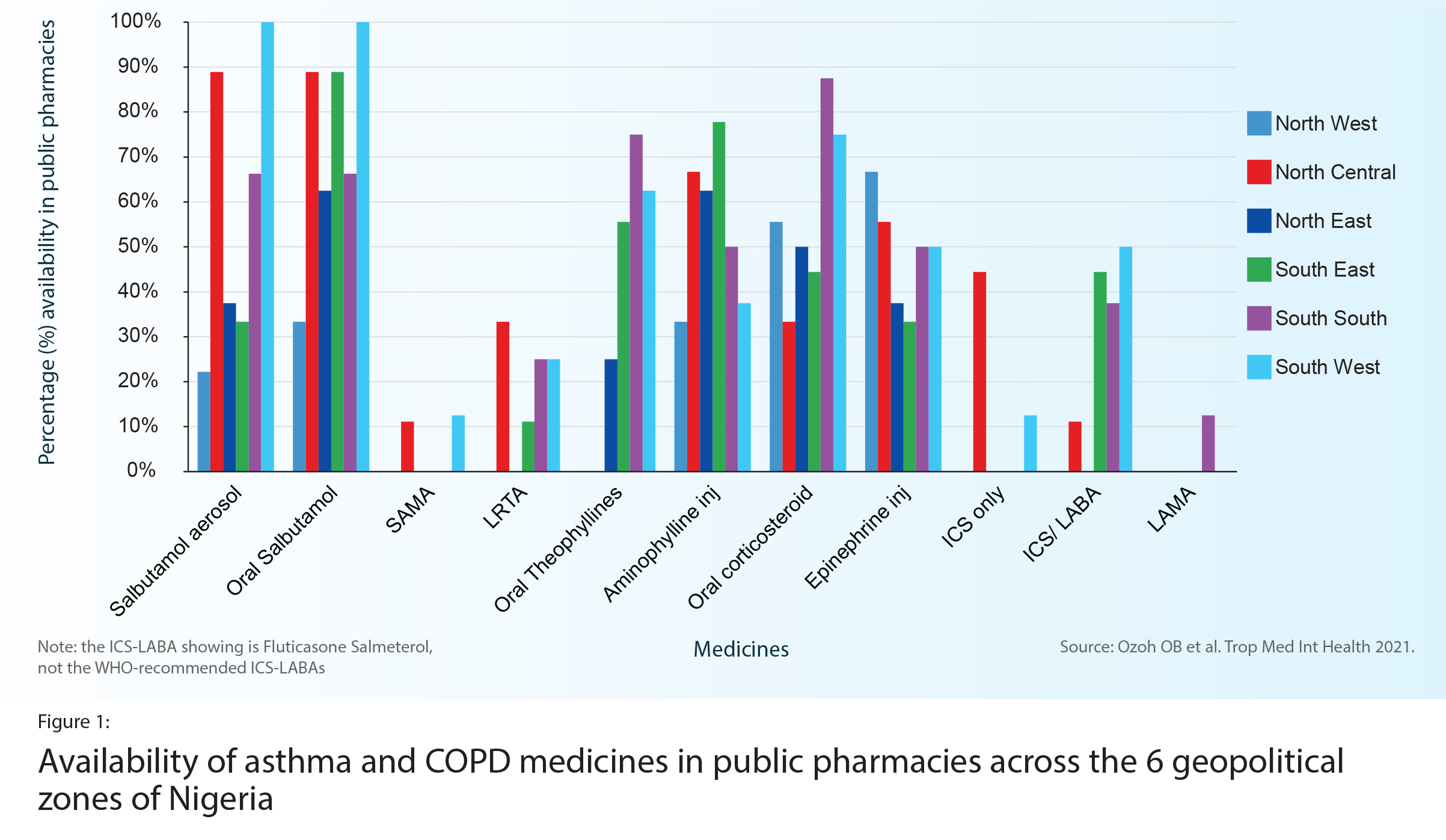
In Nigeria (Chapter 7), an onsite study in 128 public and private pharmacies found that availability of the WHO-recommended asthma medicines was far below the WHO voluntary target of 80% (Figure 1). ICS were available in only 16% of public pharmacies. One ICS-LABA was available in 48% of public pharmacies. The most available and affordable medicines were oral corticosteroids (in 73% of pharmacies) and oral salbutamol (in 72%), yet these products are not recommended for long-term asthma management. More expensive originator brands dominated more affordable generics. Private pharmacies, where costs are higher, had much higher availability than public pharmacies.
Multi-country survey in the African Region
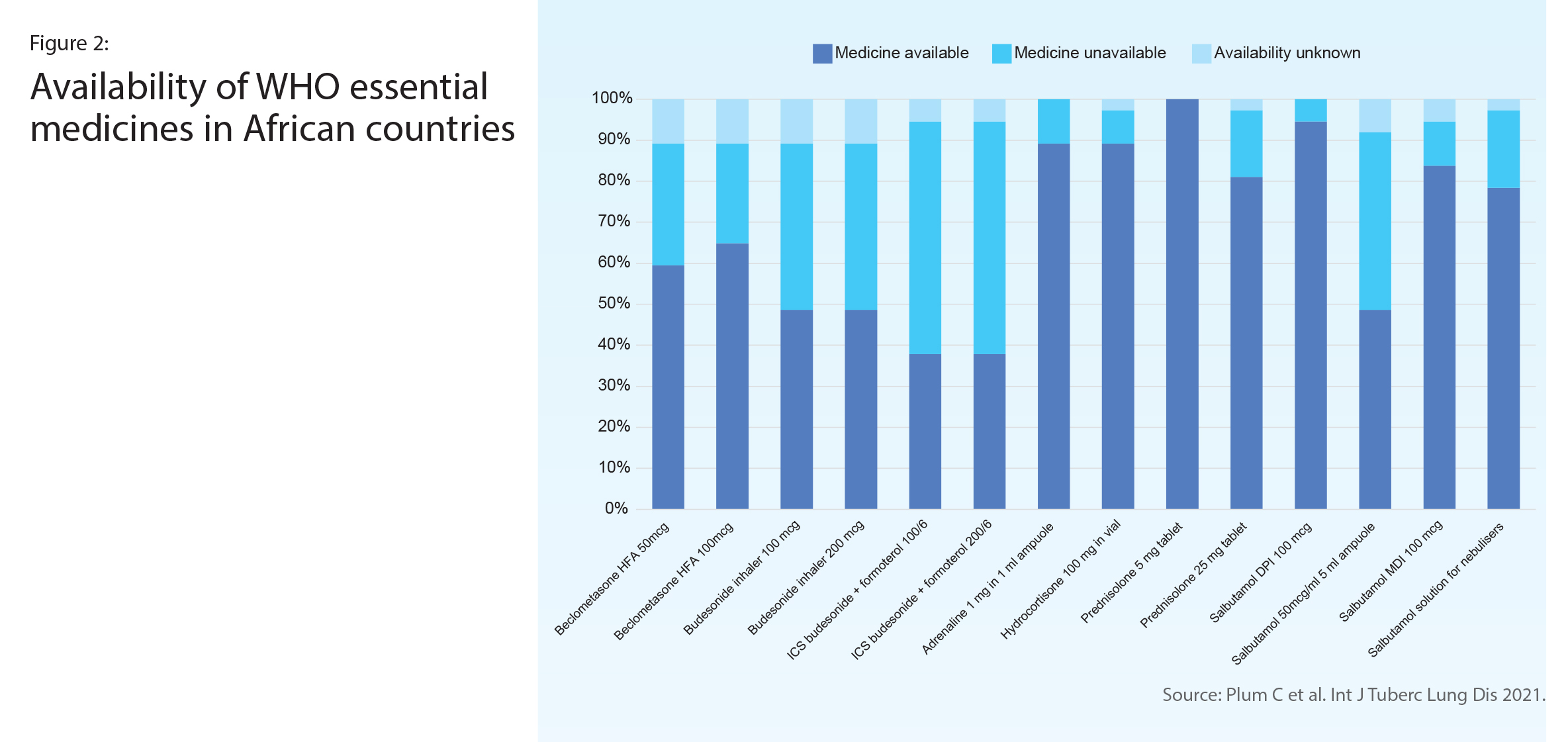
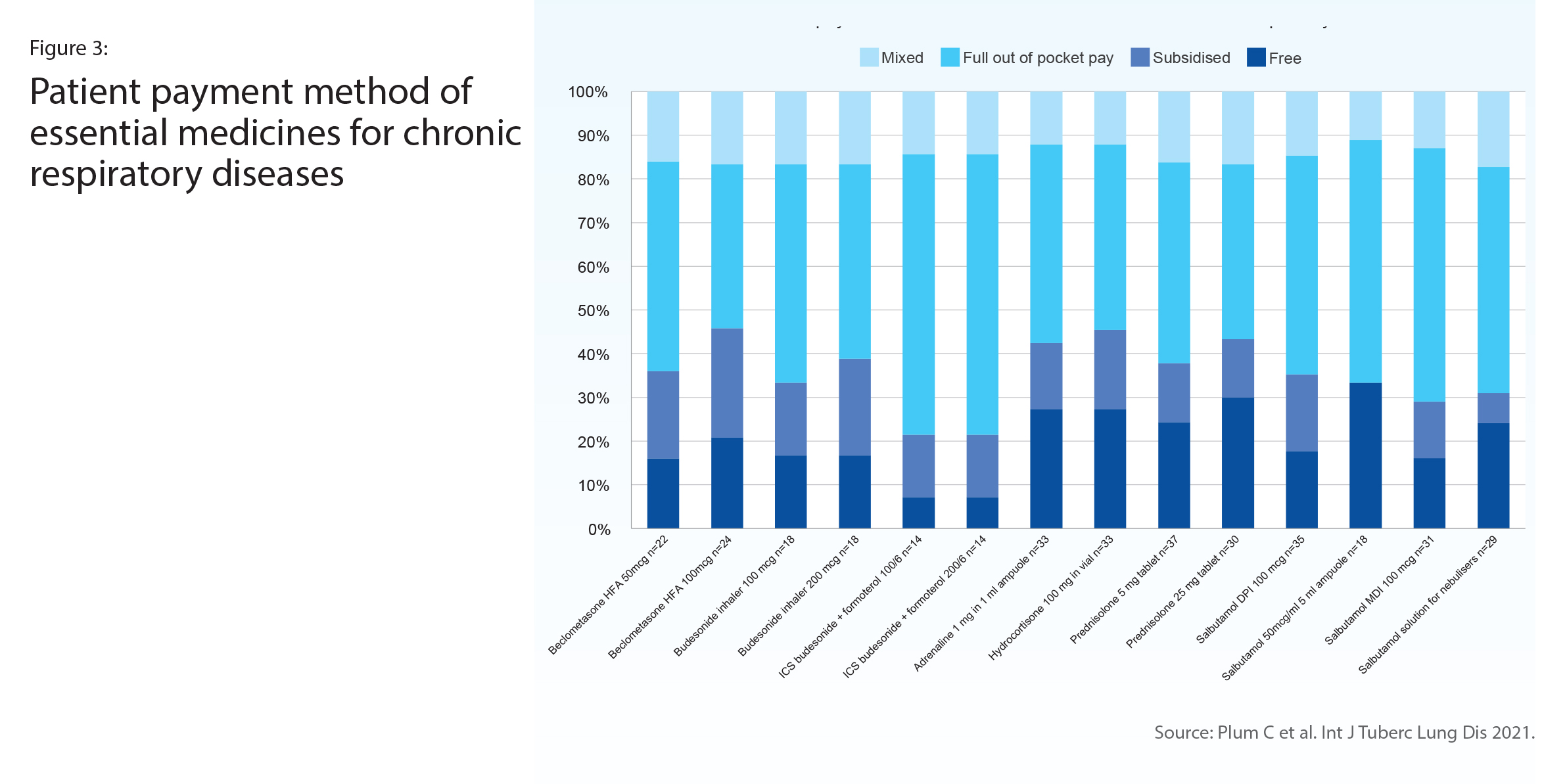
A survey of 37 respiratory specialists representing 13 countries in Africa reported on the availability in their health facility of WHO-recommended asthma and chronic obstructive pulmonary disease (COPD) medicines (Figure 2). The least available was the ICS-LABA combination inhaler, which was also the medicine most often paid out-of-pocket (Figure 3). About half reported that an ICS was available, however in half of those availability was often interrupted. Only oral corticosteroids and oral salbutamol reached the 80% availability target set out in WHO’s Global Action Plan for NCDs.
Action for improved access
Across these three studies, the reasons reported by the survey respondents and researchers for such poor availability and affordability included: prohibitive cost; WHO-recommended asthma medicines not being on the country’s essential medicines list (EML); outdated national treatment guidelines; lack of knowledge by prescribers about WHO-recommended asthma medicines and long-term asthma management; and pharmacies not stocking ICS medicines, partly because they are rarely prescribed.
It is unfortunate that people have not been benefitting from access to essential medicines such as the ICS-LABA combination inhaler. The ICS-LABA (budesonide-formoterol) serves as both a reliever and controller, which can enhance adherence (Chapter 13). However, this product has not yet made it onto the EML of Nigeria, Gambia or most of the other African countries in the above study. These three surveys reflect the same poor availability of essential asthma medicines reported in earlier surveys by he International Union Against Tuberculosis and Lung Disease (2004-2011) and the Global Asthma Network (2014). Global and country-level action and advocacy needs to be revitalised (see Table). An important step for WHO would be updating the asthma treatment recommendations in its package of essential non-communicable disease interventions for primary health care (PEN) to reflect the combination inhalers (ICS-LABA) included in the WHO Essential Medicines List.
Conclusion
Many countries still do not have the WHO-recommended essential asthma medicines on their EML, and many are not providing the medicines free or subsidised for patients, especially in low- and middle-income countries. This situation creates barriers for patient access to medicines and is likely to be perpetuating the under-prescription and underuse of ICSs and the combination inhaler. Regular international monitoring may prompt countries to work harder towards WHO’s 80% availability target for NCD essential medicines and the overarching aim of Universal Health Coverage. Monitoring should include not only the availability, pricing and affordability of essential asthma medicines. It should also report on the inclusion, or not, of essential asthma medicines in policies that are fundamental for improving access: for example, each country’s EML, asthma management guidelines and medicine reimbursement lists.